Device Return Handling Instructions
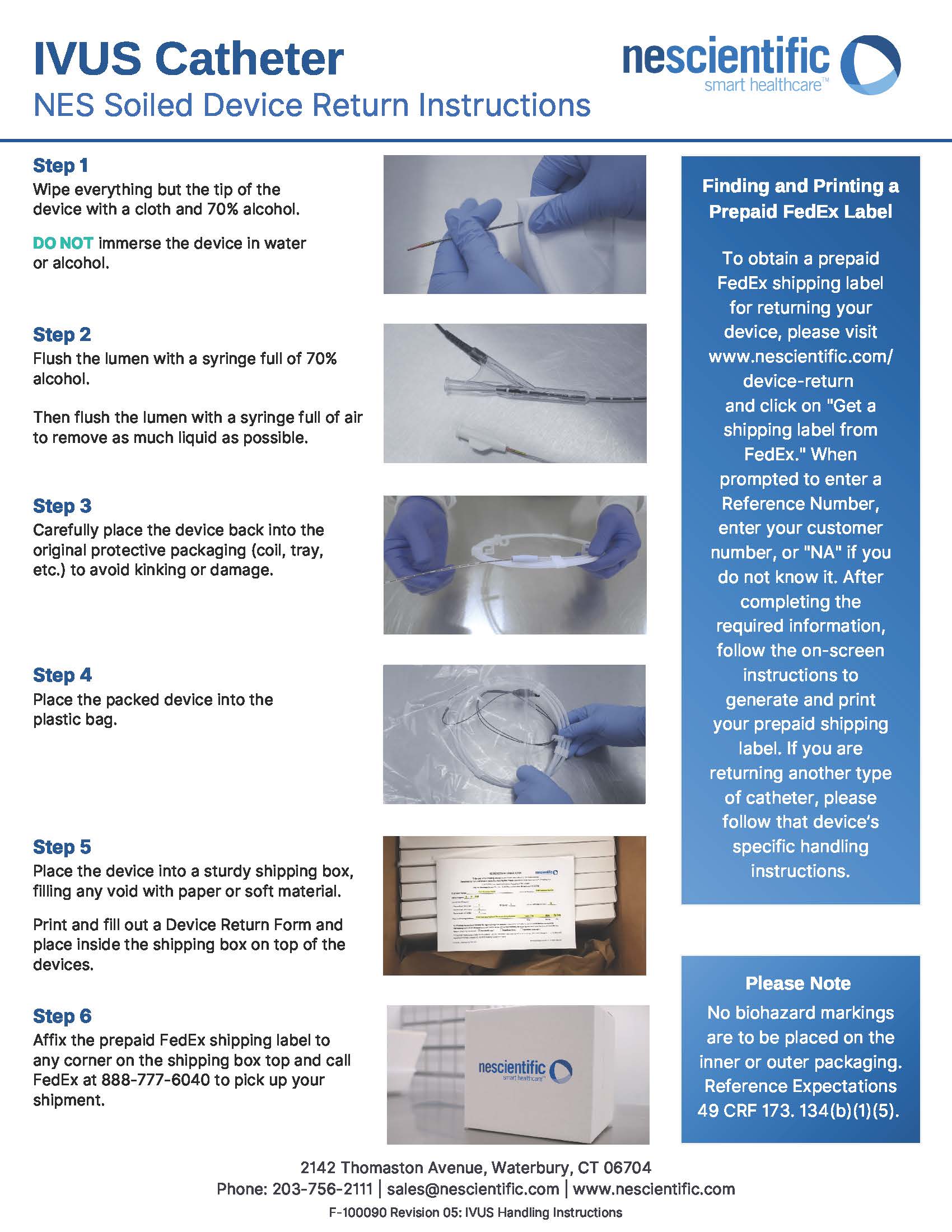
IVUS Handling Instructions

VNUS Handling Instructions

Turbo Device Handling Instructions
Instructions for Use
Quality Policy
At NEScientific, we are committed to excellence in every aspect of our work. We prioritize compliance with all regulatory requirements and continuously maintain the effectiveness of our Quality System to deliver products of the highest standards. This commitment is achieved through effective management and the dedication of our exceptional team.
Our quality-focused approach ensures regulatory and compliance standards are met, enabling us to provide safe, cost-effective, and reliable healthcare solutions. By setting and reviewing clear quality objectives, as outlined in the NEScientific Quality Manual, we ensure that our Quality System remains robust and effective, supporting our mission to enhance healthcare for both providers and patients.
Medical Device Single Audit Program (MDSAP)
At NEScientific, we proudly participate in the Medical Device Single Audit Program (MDSAP), reinforcing our commitment to quality and consistency at every stage. Successful partnerships require trust, and MDSAP certification demonstrates our dedication to delivering safe, reliable, and compliant solutions.
By adhering to the rigorous requirements of MDSAP and ISO 13485:2016, we ensure excellence through:
- Comprehensive Design History: Maintaining a detailed record for each manufactured device.
- Validated Contamination Control: Ensuring sterile medical devices meet strict contamination control standards.
- Complaint Monitoring and Reporting: Implementing robust systems for handling complaints and regulatory reporting.
- Qualified Personnel and Training: Defining specific skills for quality management roles and verifying ongoing training effectiveness.
- Risk Management Feedback: Using production and post-production insights to enhance risk management programs.
- Quality System Validation: Conducting thorough validation of quality management system software.
Our participation in MDSAP reflects NEScientific’s unwavering commitment to delivering the highest quality in medical device reprocessing while supporting our partners in achieving their goals.
What This Means for Valued Partners
“MDSAP is another part of how Northeast Scientific has reinforced its position in the market for setting the standard for quality and consistency in the reprocessing space. We firmly believe that we can give the doctors who purchase our reprocessed devices peace of mind, knowing that our Quality system meets the stringent requirements of ISO 13485 Certification through MDSAP to get them devices at a huge cost savings,”
– Matt Farley, Director of Product Development and Interim Director of Quality and Regulatory