
Save hundreds of thousands each year with reprocessed IVUS and atherectomy catheters from Northeast Scientific.
Are you throwing away a significant cost-saving opportunity?
It’s common practice to assume that IVUS and atherectomy catheters are a single-use device. However, reprocessing is a safe, sustainable, and cost-effective option. Our reprocessed catheters have undergone an extensive disinfecting and sanitizing process and are FDA 510(k) cleared. Physicians are saving up to 30% on their IVUS and atherectomy catheters by partnering with Northeast Scientific.
An exciting opportunity to increase your bottom line
Is your practice affected by year-over-year reduced reimbursements?
Northeast Scientific presents a great opportunity to save considerable costs.
Current purchasing from Original Equipment Manufacturer
Total monthly IVUS needs

25 DEVICES
at $800 per device
Total monthly Atherectomy needs

25 DEVICES
at $1,300 per device
Tax rate: 6.5%Total cost per month: $55,912.50
Purchasing 1/3 of your device needs from Northeast Scientific
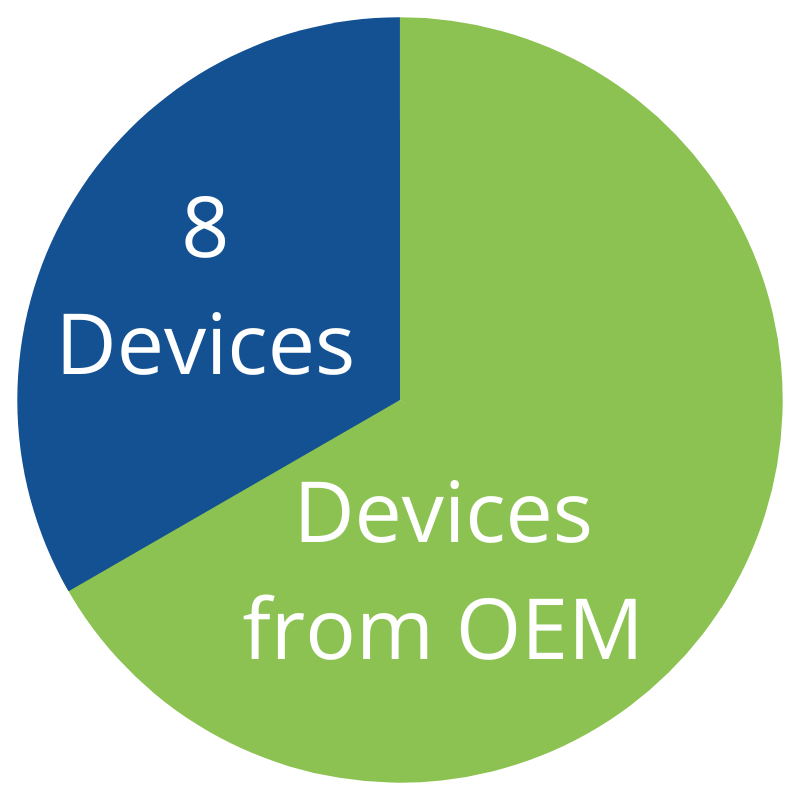
NES IVUS cost per device
$475
Total: $3,800

NES Atherectomy cost per device
$950
Total: $7,600
New monthly purchasing costs

Total OEM Monthly Cost: $55,912.50Total Combined NES & OEM Monthly Cost: $49, 420.50
Total monthly savings: $6,492
Total yearly savings: $77,904
Northeast Scientific has saved thousands of dollars for our clients. See how our savings adds up over the years.
In 2023, our customer base collectively saved over $9 million by purchasing a combination of catheters from Northeast Scientific and the OEM, versus purchasing exclusively from the OEM.
Current Customer Savings Case Studies
* Pricing is based on purchasing volume *
Single-doctor practice saves $15,000 on IVUS & atherectomy devices over six months
Doctor originally bought 50 of each device from the OEM, now purchases 20 of each device from Northeast Scientific
Multi-site practice saves more than $100,000 in IVUS & atherectomy devices over six months
Practice originally bought 120 of each device from the OEM, now purchases 45 of each device from Northeast Scientific
NES devices are reprocessed through robust procedures and inspections cleared by the FDA 510(k) process, with all steps being controlled by our ISO 13485 certified Quality System.

Northeast Scientific reprocessed devices undergo procedures cleared by the FDA through the 510(k) process.
Reprocessed devices purchased from Northeast Scientific have been shown to be substantially equivalent to the original device in safety and efficacy.

After clinical use, devices are carefully packaged according to NEScientific Handling Instructions to minimize potential damage and maximize reprocessing success. Customers then schedule a FedEx® pickup using pre-paid labels, ensuring safe and efficient delivery to NEScientific.

Upon arrival at NEScientific, devices undergo an initial inspection to identify any issues or irreparable damage that could prevent reprocessing. While NEScientific continuously enhances its reprocessing capabilities, strict quality control guidelines ensure only acceptable devices move forward in the process.

Each device undergoes a rigorous decontamination and cleaning process tailored to its specific type. Throughout the production cycle, devices are meticulously inspected at multiple stages to ensure quality and compliance.

NEScientific’s proprietary technology enables highly effective cleaning methods, preparing devices for safe clinical reuse. Each device is tracked with a detailed history record to ensure every step of the process is completed with precision.

Devices undergo individual inspection and functionality testing. Once all requirements are met, each device is carefully pouched, labeled, and prepared for sterilization.

After completing the reprocessing cycle, devices are sterilized using 100% ETO. Post-sterilization testing ensures all requirements are met, followed by a final inspection to confirm readiness for sale.

The NEScientific Quality team verifies that each device meets all required internal specifications. Once approved, devices are added to inventory and made available for purchase by customers.
Quality is Priority at Northeast Scientific
Northeast Scientific’s Quality Management System has been audited and certified to FDA CFR 820 and ISO 13485:2016 under MDSAP by Intertek.
Defined requirements for a safe and effective Quality System, which governs all NES processes.
Change the color to match your brand and more.
Maintenance of a comprehensive design history file for each manufactured device, including requirements and controls for any design changes.
Change the color to match your brand and more.
Measurement, analysis, and improvement for complaint handling, equipment, processing, and regulatory reporting.
Change the color to match your brand and more.
A clear definition of specific skills and experience of quality management personnel, plus verification of ongoing training effectiveness.
Change the color to match your brand and more.
Formal processes for obtaining and incorporating production and post-production feedback into risk management programs.
Change the color to match your brand and more.
What Our Partners Are Saying
Discover how healthcare providers are benefiting from our trusted reprocessing solutions—delivering quality, savings, and sustainability in every device.
“I would recommend Northeast Scientific to anyone who is looking for ways to meaningfully reduce their expenses while maintaining very high levels of quality.”
— Michael D’Aquila
COO, U.S. Vascular Management Group
On the heels of the success of our IVUS catheter program, we will be rolling out additional reprocessed device programs, allowing you even more savings over time. Stay tuned for more information.

Frequently Asked Questions
Once. Currently Northeast Scientific only reprocesses IVUS or atherectomy catheters for one cycle.
Please send back ALL post procedure catheters, OEM devices and those purchased from NEScientific, using the prepaid labels and supplies NEScientific provides.
Save the catheter and send your Clinical Sales Specialist an email detailing the issue and NEScientific will send you a Return #. You will be issued a credit or replacement after the device is received and inspected.